
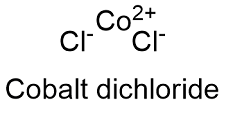
Why? Everyone knows that a metal and a non-metal form ionic bonds and have giant structures. The data do not require a description of CrO/sub 2//sup 2 +/ as other than a superoxochromium(III)ion.COBALT CHLORIDE - Molecule of the Month June 2016 - HTML-only versionĬOBALT CHLORIDE A drug used to dope racehorses that's also a water indicator. The Lewis basicity of O/sub 2//sup -/ for Cr/sup 3 +/ is discussed on the basis of comparisons to cobalt(III)-ammine complexes, other (H/sub 2/O)/sub 5/CrX/sup 2 +/ complexes, and acid ionization constants.

The latter, although kinetically unimportant, is suitable for thermodynamic analysis. The equilibrium constant for homolysis of CrO/sub 2//sup 2 +/ is K/sub hom/ = 1.6 x 10/sup -12/ M and that calculated from the thermodynamic data for heterolysis (CrO/sub 2//sup 2 +/ in equilibrium Cr/sup 3 +/ + O/sub 2//sup -/) is K/sub het/ = 3 x 10/sup -8/ M. A structure of the activated complex for this pathway is proposed. It is characterized by a rate constant 6.0 +/- 0.9 M/sup -1/ s/sup -1/ at 25/sup 0/C (.delta.H/sup double dagger/ = 12.0 +/- 1.7 kcal mol/sup -1/. A second pathway, which yields HCrO/sub 4//sup -/, proceeds by a bimolecular reaction between two CrO/sub 2//sup 2 +/ ions. The resulting Cr/sup 2 +/ then reacts very rapidly with CrO/sub 2//sup 2 +/, with k = 8 x 10/sup 8/ M/sup -1/ s/sup -1/, more » estimated by kinetic modeling of the effect of (O/sub 2/). One pathway for decomposition consists of bond homolysis, CrO/sub 2//sup 2 +/. The cation (H/sub 2/O)/sub 5/CrO/sub 2//sup 2 +/ has a considerable lifetime in aqueous solution in the absence of Cr/sup 2 +/. Rare earth oxides have been halogenated and vapor transported using Al/sub 2/Cl/sub 6/ or Al/sub 2/Br/sub 6/. At 650/sup 0/K the volatility enhancement of Sm(II) has been estimated to be approx.10/sup 10/. For the reaction SmCl/sub 3/(s) + 3/2Al/sub 2/Cl/sub 6/(g). more » Thermodynamic considerations suggest that the SmAl/sub 3/Cl/sub 12/ is the predominant vapor species formed by the trivalent samarium. Spectrophotometric measurements have been used to investigate the partial pressures of the vapor complexes at different Al/sub 2/Cl/sub 6/ pressures. The spectra of the red-brown Sm/sup II/-Al-Cl gaseous complex(es) showed high-intensity, broad bands which are attributed to 4f. The spectra of the pale yellow Sm/sup III/-Al-Cl gaseous complex(es) were characteristic of the f. The electron absorption spectra of vapor-phase compounds formed by reacting SmCl/sub 3/(s) and SmCl/sub 2/(s) with gaseous Al/sub 2/Cl/sub 6/ have been measured at temperatures up to 800/sup 0/K and total pressures up to 13 atm. delta.H/sup double dagger/ = 7.8 +- 0.1 kcal mol/sup -1/. Line-shape analysis of the /sup 133/Cs resonance at various temperatures gave rate parameters for the decomplexation of the 1:1 complex as follows. The thermodynamic functions for the second step are: (.delta.G/sup 0//sub 2/)/sub 298/ more » = -2.58 +- 0.02 kcal mol/sup -1/. 10/sup 6/ at 298/sup 0/K) followed by the addition of a second ligand molecule to form a sandwich compound, Cs/sup +/(18C6)/sub 2/ (K/sub 2/ = 44 +- 2 at 298/sup 0/K). A study of the /sup 133/Cs chemical shift as a function of crown/Cs/sup +/ mole ratio showed that the complexation reaction occurs in two steps-formation of a stable 1:1 complex (K/sub 1/ approx. =, number = ,Ĭesium-133 nuclear magnetic resonance was used as a probe of the interaction of Cs/sup +/ with the macrocyclic polyether 18-crown-6 (18C6) in pyridine solutions.


 0 kommentar(er)
0 kommentar(er)
